What Is T-DM1 And What Is It Used For?
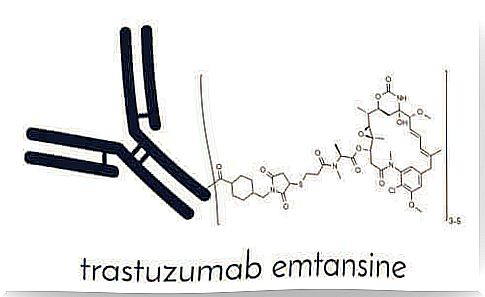
T-DM1 is a new and unique selective antibody drug combination. It is a medicine approved by the European Medicines Agency (EMA) for the treatment of advanced HER-2-positive breast cancer. HER-2 is a “epidermal growth factor receptor 2” protein that promotes the growth of cancer cells. It is sold as a medicine called Kadcyna.
T-DM1 consists of two components. The first is a known drug called trastuzumab, which is an anti-HER-2 antibody drug. The second ingredient is the cytotoxic DM-1 antimicrotubule molecule. Cytotoxic antimicrotubule refers to its ability to inhibit microtubule synthesis in cell division. We explain this mechanism in more detail below.
Efficacy of T-DM1

18-20% of prevalent cases of breast cancer are HER-2-positive. Thus, amplification of the HER-2 oncogene is found in them. Prior to the development of specific anti-HER-2 therapies, HER-2-positive patients had a much worse prognosis than others. An oncogene is a gene that leads to the formation of cancer in a cell because it has the ability to cause a mutation or change.
Thanks to the development of trastuzumab approved in 2000, the poor prognosis of HER-2-positive breast cancer cases compared to HER-2-negative ones improved. In 2014, another HER-2 antibody drug called pertuzumab was launched. The latter drug prolongs the overall overall survival in the first treatment of advanced HER-2-positive breast cancer.
Another treatment for advanced HER-2-positive breast cancer was the only treatment approved to date that is combination chemotherapy with cabecitabine and lapatinib, a tyrosine kinase inhibitor of HER-2 / EGFR proteins.
The EMILIA registry study compared T-DM1 with lapatinib and cabecitabine in patients with a type of breast cancer that had previously been treated with trastuzumab and taxane. The results of this study showed an increase in progression-free survival, overall survival, as well as better tolerability of side effects and a significant delay in symptoms until progression to T-DM1 treatment.
How T-DM1 exerts its effects on the body
As mentioned above, T-DM1 is a combination of two drugs (trastuzumab and DM1). T-DM1 combines the mechanisms of action of these two drugs:
- Like trastuzumab, T-DM1 is able to bind HER-2 protein and inhibit the growth of cancer cells that are amplified by this growth factor.
- It also provides a mechanism of action for DM1 and is therefore able to bind tubulin.
Because it inhibits tubulin, it does not allow cancer cells to divide, eventually leading to cell death caused by apoptosis. In vitro cytotoxicity experiments show that DM1 is 20-200 times more potent than vinca taxanes and alkaloids.
Side effects of T-DM1
Side effects are unwanted and unintended events that patients can expect to receive when starting treatment.
In this case, the most reported side effects are nausea, fatigue, and headache. They affected less than 25% of patients. Thus, experts evaluated the safety of T-DM1 in a total of 1,871 breast cancer patients in various clinical trials. They found that the most common serious reactions were:
- Haemorrhage
- Shortness of breath
- Pain in bones and muscles
- Stomach ache
- Thrombocytopenia
- Vomiting
Summary
Data reported from the studies indicate that T-DM1 is a very important advance in the treatment of advanced HER-2-positive breast cancer. It increases the survival of patients who have taken trastuzumab before.
Despite these advances, HER-2-positive cancer is still incurable. It is therefore essential to find new, more tolerable and more effective treatments. It is important to continue actively researching breast cancer.